Topmind Medical is a professional innovative medical supplier in China,devoting to R&D, manufacturing, marketing for ranges of minimally invasive interventional medical devices especially guidewire and catheter, which will be widely used in cardiology, neurology, anesthesiology, oncology, nephrology, urology, laparoscopy, hepatobiliary pancreatic surgery, ICU, pain management etc.
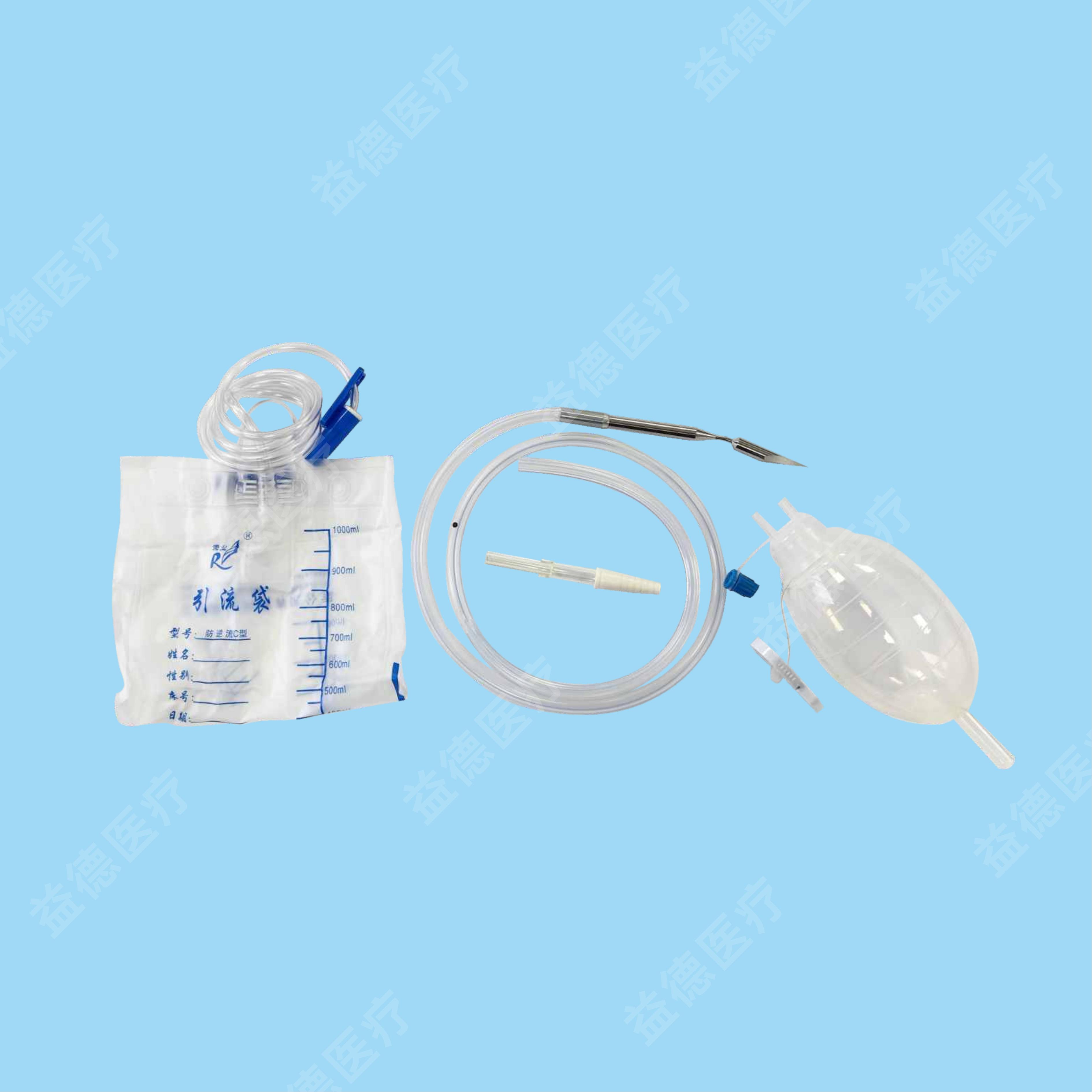
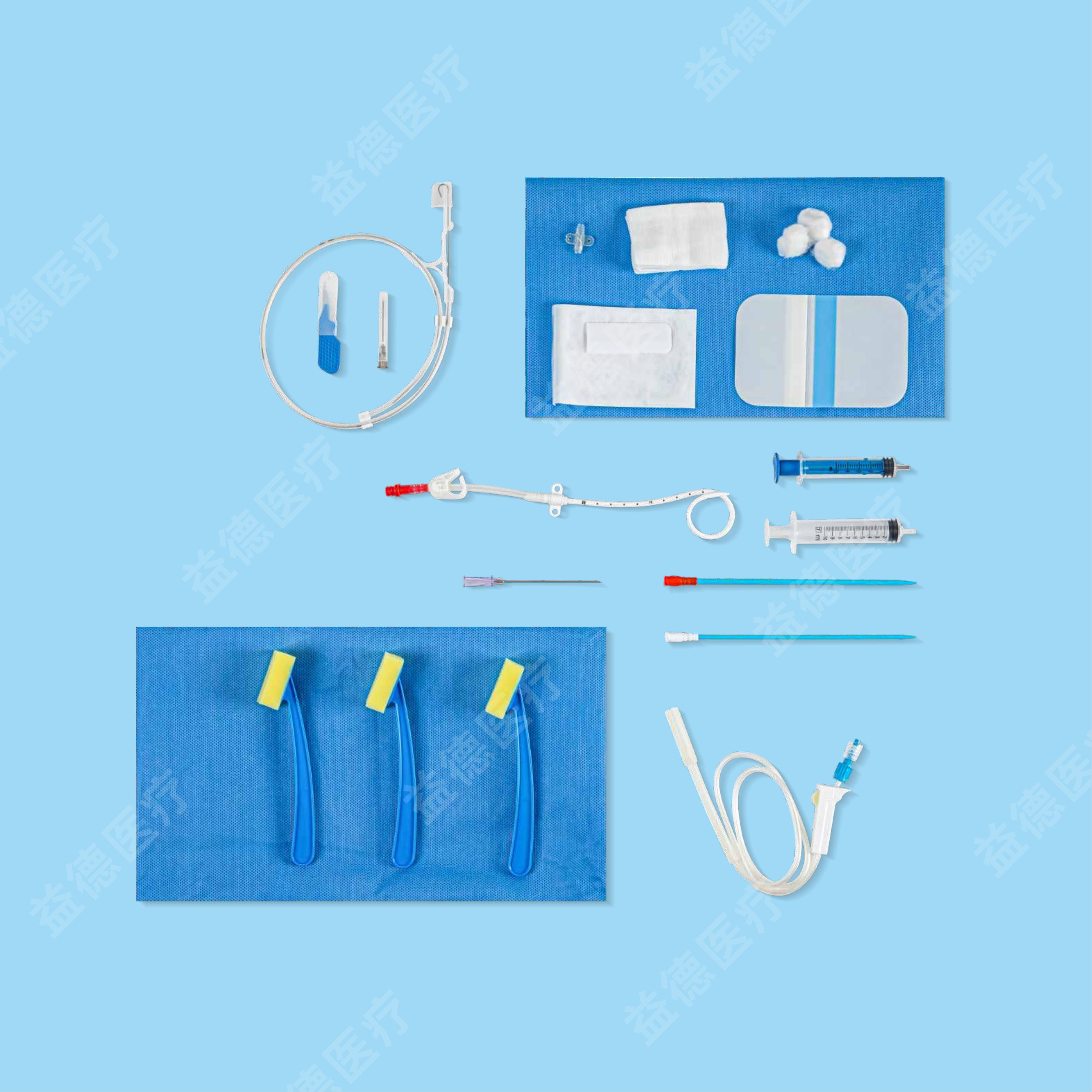
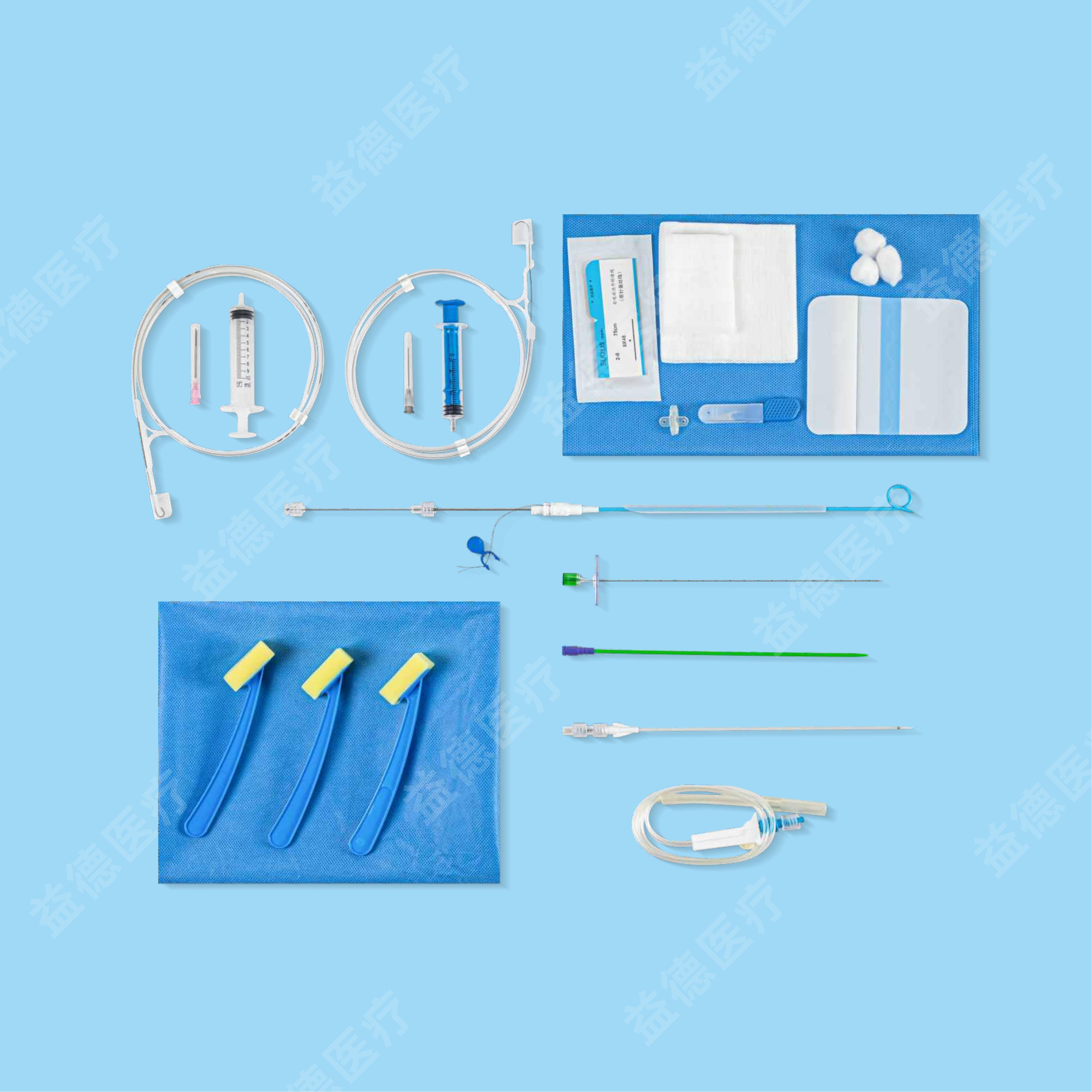
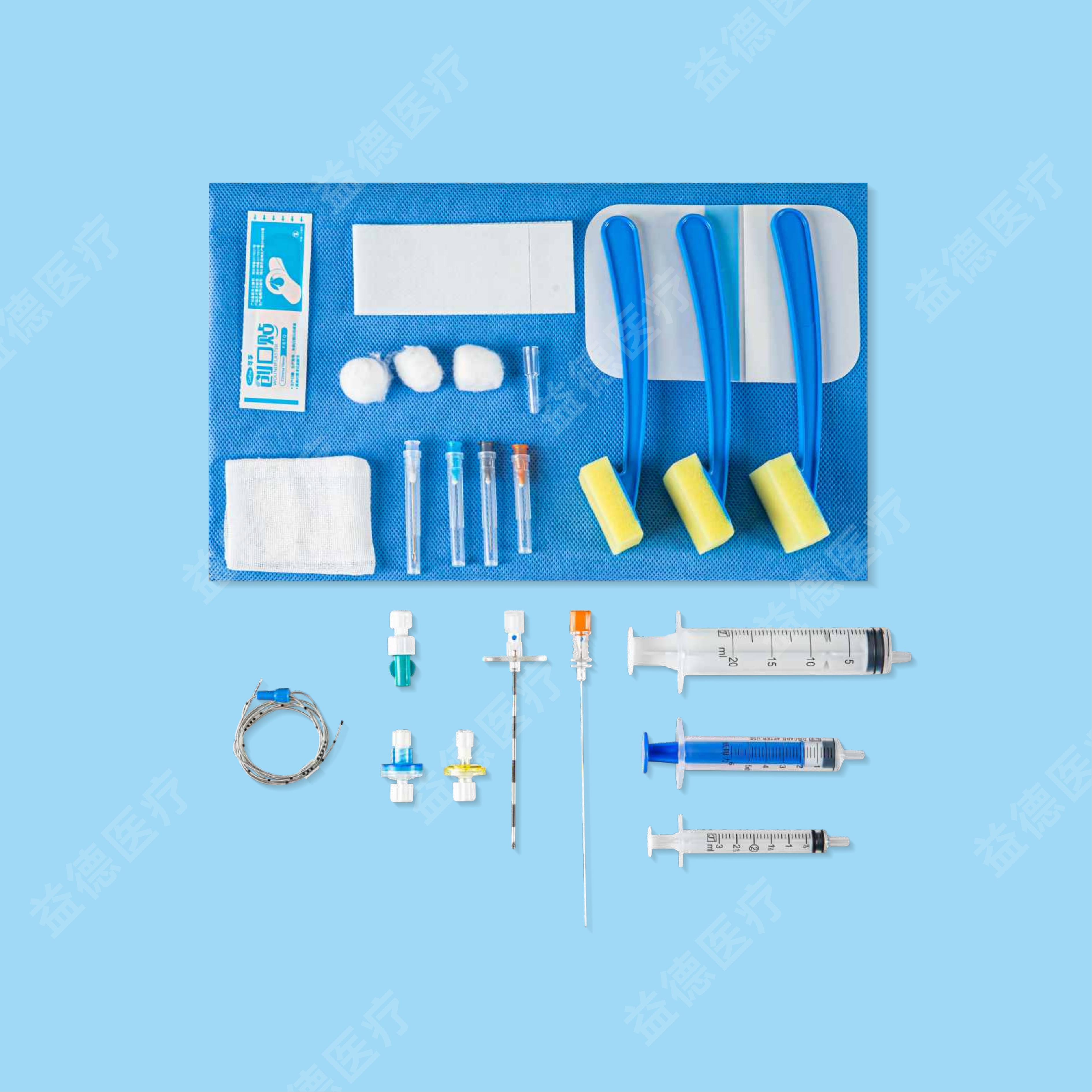
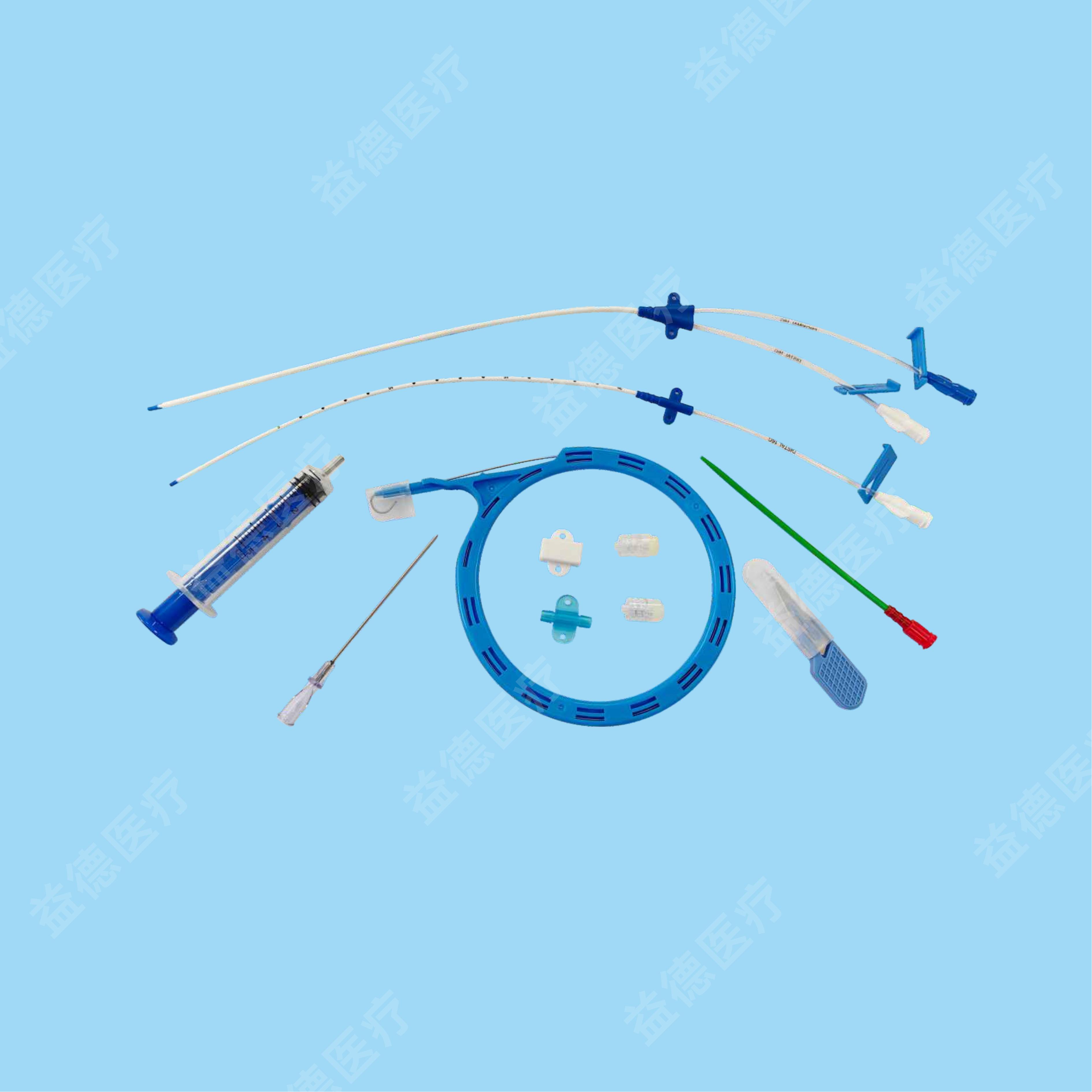
The products Topmind Medical provide are include but not limited as follow: Central Venous Catheter, Hemodialysis Catheter, PICC, Spring Coil Reinforcement Epidural Anesthesia Catheter, Biliary Drainage Catheter, Thoracoadominal Drainage Catheter,Negative Pressure Drainage Catheter, Angiographic Catheter, Brain Surgery Dilated Balloon Catheter, Ureteral Stents, Seldinger Guidewire, PTFE coated Guidewire, Hydrophilic Guidewire, Zebra Guidewire, Movable Guidewire, PTCA guidewire etc.
With more than 15 years experience of R&D and manufacturing series of vascular and non-vascular guiewire and catheter, Topmind teams are innovative to creating and assembling many key techniques processing equipment by themselves such as but not limited to the below machines: guidewire straightening machine , guidewire ”J-tip” bending machine, guidewire and catheter hydrophilic coating machine, catheter tip-formning machine, catheter side-hole drilling machine etc.
Topmind Medical keeps in mind the mission of "make people healthier, make life happier", in line with the business philosophy of "customer-centric, value contribution oriented" and the quality policy of "the product is symbol of personal quality, the quality is equal to human life", stick to the development route of independent R&D and innovation, applies for a number of product process inventions and utility intellectual property patents, and strives to provide more safe and more effective innovative medical device products for customers.
-
 Over 10 years of industry experience
Over 10 years of industry experience -
 Obtained EU CE certification
Obtained EU CE certification -
 High quality products and services
High quality products and services -
 Compliant with ISO 13485:2016 standard
Compliant with ISO 13485:2016 standard
-
Invitation Letter | From September 26th to 29th, the 92nd China International Medical Equipment (Autumn) Expo, looking forward to your visit!
 2025-09-17The 92nd China International Medical Equipment (Autumn) Fair (CMEF) will be held from September 26 to 29, 2025 at the China Import and Export Fair Complex (Guangzhou). At that time, Guangdong Yide Med
2025-09-17The 92nd China International Medical Equipment (Autumn) Fair (CMEF) will be held from September 26 to 29, 2025 at the China Import and Export Fair Complex (Guangzhou). At that time, Guangdong Yide Med -
Won the ISO13485 quality management system certification
 2025-09-11Topmind Medical successfully passed the ISO13485 quality management system certification in September 2025, which is a passport to enter the international medical device market, marking that the compa
2025-09-11Topmind Medical successfully passed the ISO13485 quality management system certification in September 2025, which is a passport to enter the international medical device market, marking that the compa -
关于医疗器械保质期的常见问题摘要
 2024-10-031、什么是医疗器械的保质期? 它指的是医疗器械在形成最终产品后能够发挥建议作用的时间段。保质期的结束是指产品的有效期。过了这个期限,医疗器械产品就可能不再具有预期的性能参数和功能。2、影响医疗器械货架有效期的因素有哪些? 外部因素主要包括。储存条件、运输条件、生产方式、生产环境、包装、原辅材料来源变化的影响、其他影响因素等。内部因素主要包括医疗器械中各原料/组分的性能;医疗器械中各原料/
2024-10-031、什么是医疗器械的保质期? 它指的是医疗器械在形成最终产品后能够发挥建议作用的时间段。保质期的结束是指产品的有效期。过了这个期限,医疗器械产品就可能不再具有预期的性能参数和功能。2、影响医疗器械货架有效期的因素有哪些? 外部因素主要包括。储存条件、运输条件、生产方式、生产环境、包装、原辅材料来源变化的影响、其他影响因素等。内部因素主要包括医疗器械中各原料/组分的性能;医疗器械中各原料/