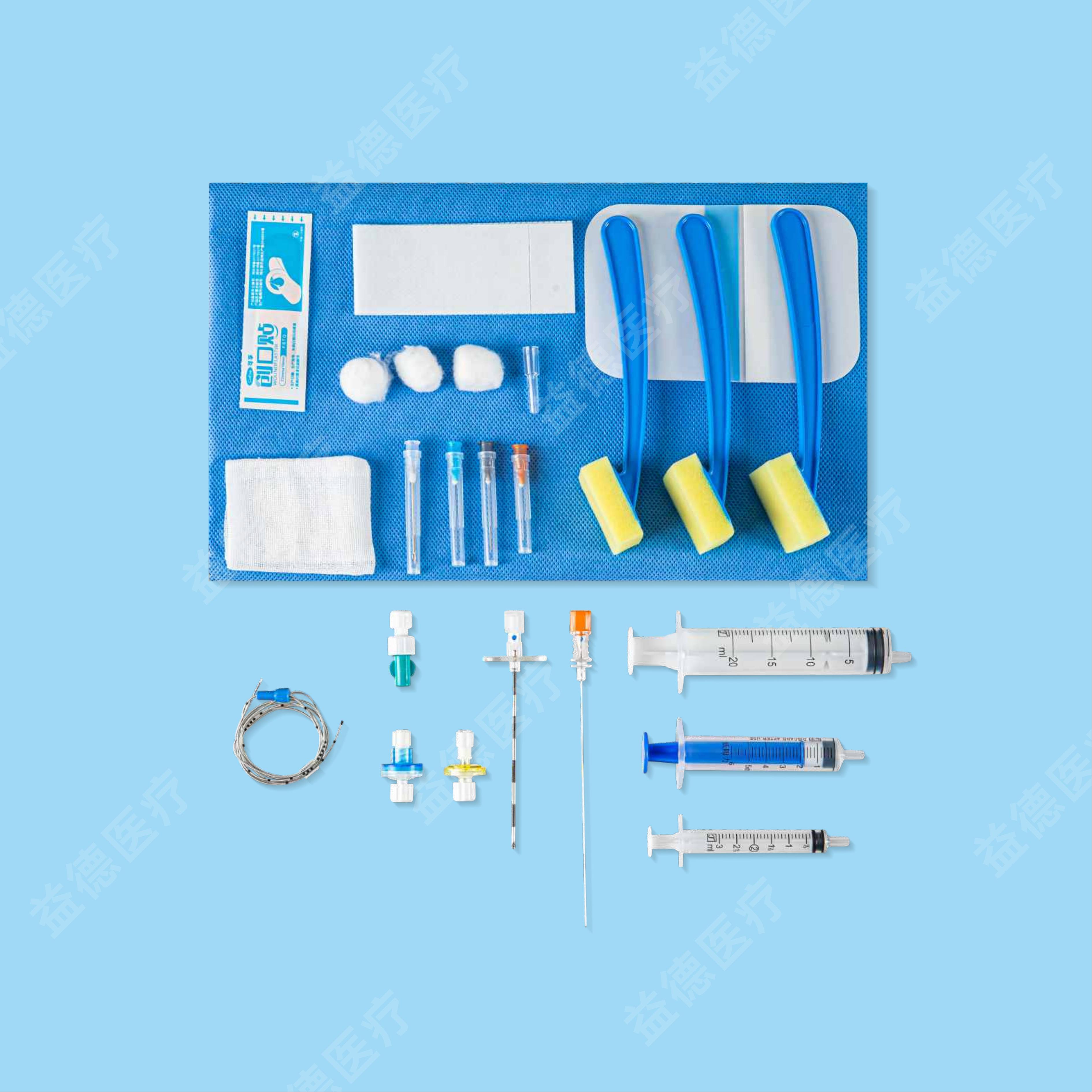
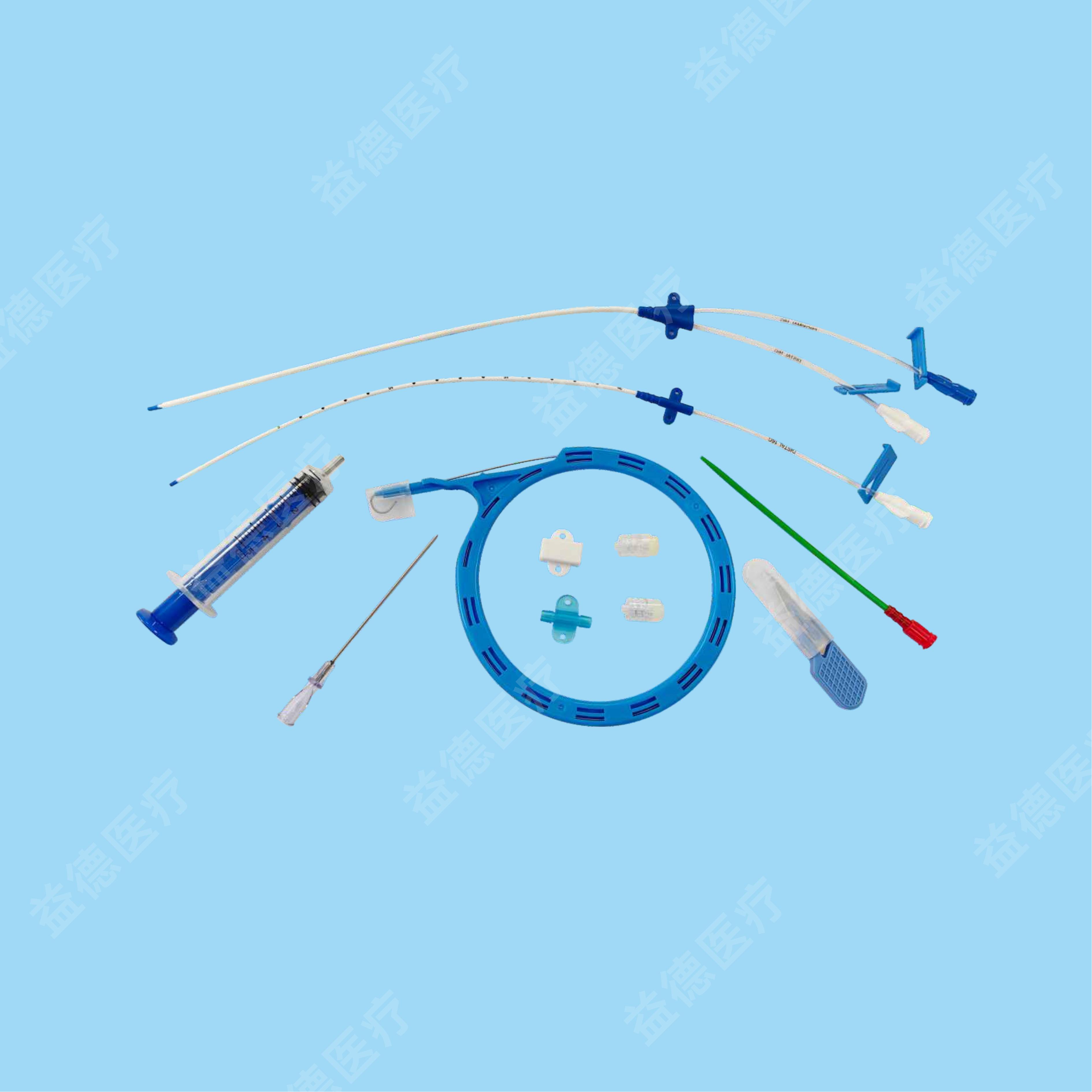
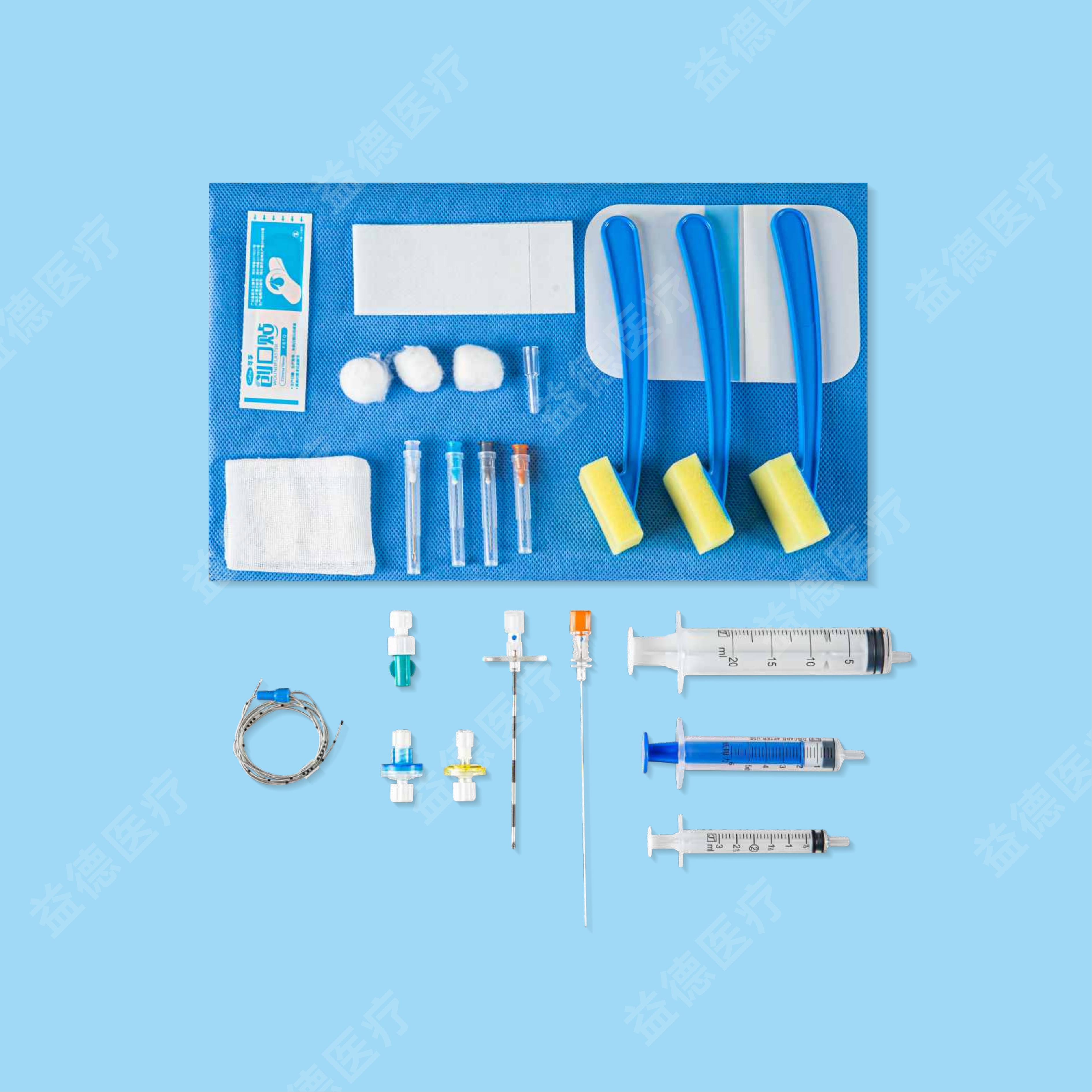
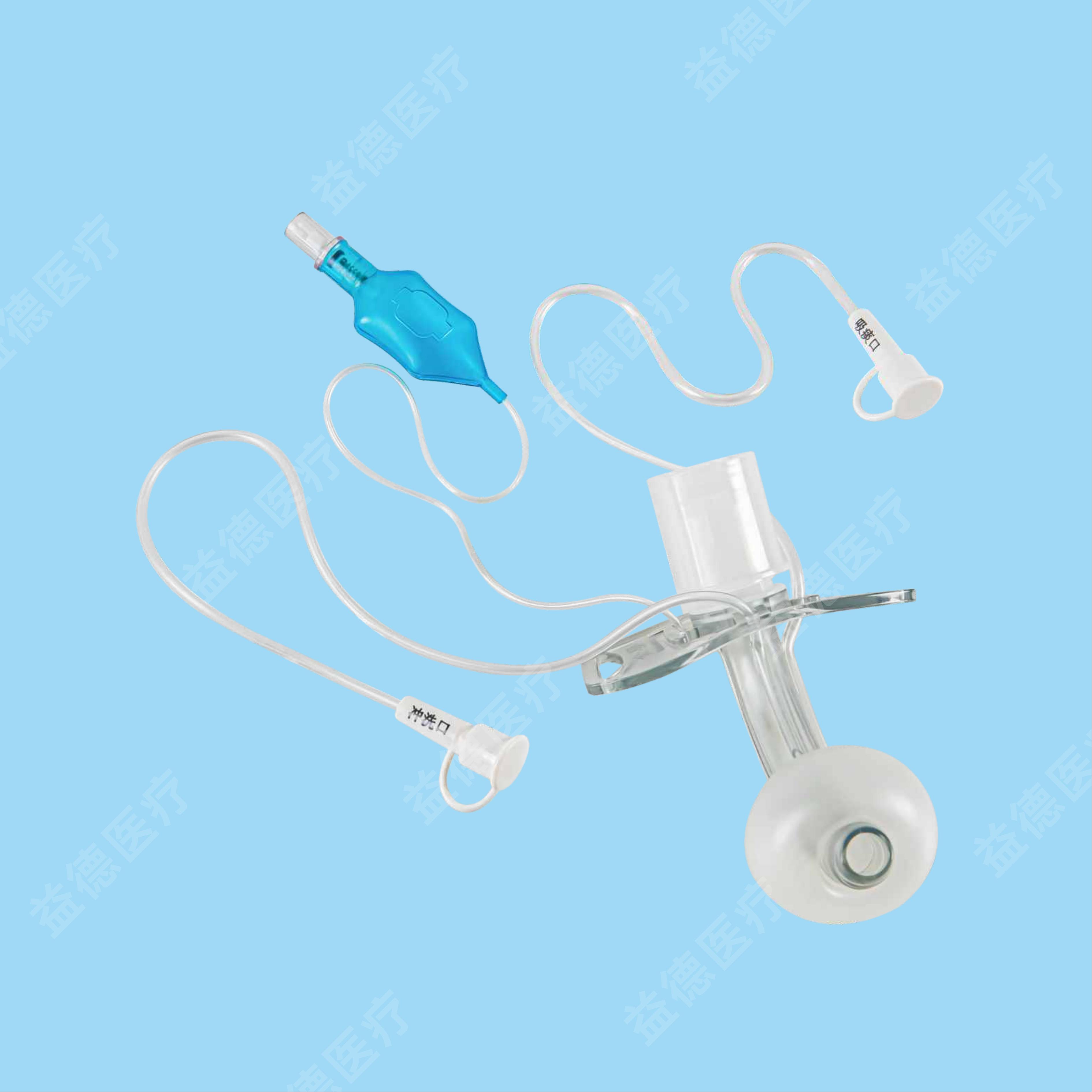
Epidural and Spinal Combined Anaesthesia Kits
- Specifications
- Product Feature
| Specification | Size |
| Epidural Anaesthsisa Kits (AS-E) | Epidural catheter diameter of 0.70/0.80mm, with open tip, front window length of 50cm and 70cm; Standard/Composite package |
| Epidural Anaesthsisa Kits (AS-E) | Epidural catheter diameter of 0.90/1.0/1.1mm, with open tip/Close tip, front window length of 50cm and 70cm; Standard/Composite package |
| Spinal Anaesthesia Kits( AS-S ) | AS-S;Standard/Composite package |
| Nerve Block Anaesthesia Kits (AS-N) | Epidural Needle 0.70*80/90mm;Standard/Composite package |
| Nerve Block Anaesthesia Kits (AS-N) | Epidural Needle 0.90*80/90mm;Standard/Composite package |
| Epidural and Spinal Combined Anaesthesia Kits (AS-E/SⅡ) | Epidural catheter diameter of 0.70/0.80mm, with open tip, front window length of 50cm and 70cm; Standard/Composite package |
| Epidural and Spinal Combined Anaesthesia Kits (AS-E/SⅡ) | Epidural catheter diameter of 0.90/1.0/1.1mm, with open tip/Close tip, front window length of 50cm and 70cm; Standard/Composite package |
1)Imported TPU materials for the catheter with good biocompatibility;
2)Different choice of open-tip,close-tip,blue soft-tip for the implantation end.
3)With observation window at the front part, middle part of the catheter, which would help doctors to make sure whether there is blood return.
4)The implantation end is soft and elastic, which will reduce the catheterization resistance, avoiding damage of the tissue.
5)The inner wall of the catheter is sustaining by spring coil, avoiding catheter bending and drug blocking.